Lewis Dot Diagram For H2o Wiring Site Resource
The Lewis structure of H2O is drawn in such a manner that the deficiency of each atom is fulfilled. Lewis Structure of H2O The Lewis structure of hydrogen and 2 oxygen atoms shows a total of eight valence electrons participate in the bond formation to form a single triatomic H2O molecule.

【4 Steps】H2O Lewis StructureLewis Structure for H2O (Water)Lewis
The Lewis structure is a visual representation of the arrangement of atoms and valence electrons in a molecule. How to Draw Lewis Structure of H2O Water (H2O) is a molecule composed of two hydrogen atoms bonded to a central oxygen atom.

H2O Lewis structure and Molecular Geometry [No1 Best Explanation
Steps of drawing H2O lewis structure Step 1: Find the total valence electrons in H2O molecule. In order to find the total valence electrons in H2O molecule, first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.). Here, I'll tell you how you can easily find.
The DangerousSounding Threat of DHMO Now I Know
Here we will first place the atoms and individual valence electrons to understand the Lewis structure of H2O step-by-step. Oxygen atoms will take a central position as Hydrogen atoms always go on the outside. So place Oxygen in the center with both the Hydrogen atoms on the side.
Draw Step By Step The Lewis Structure For Water (H2O)
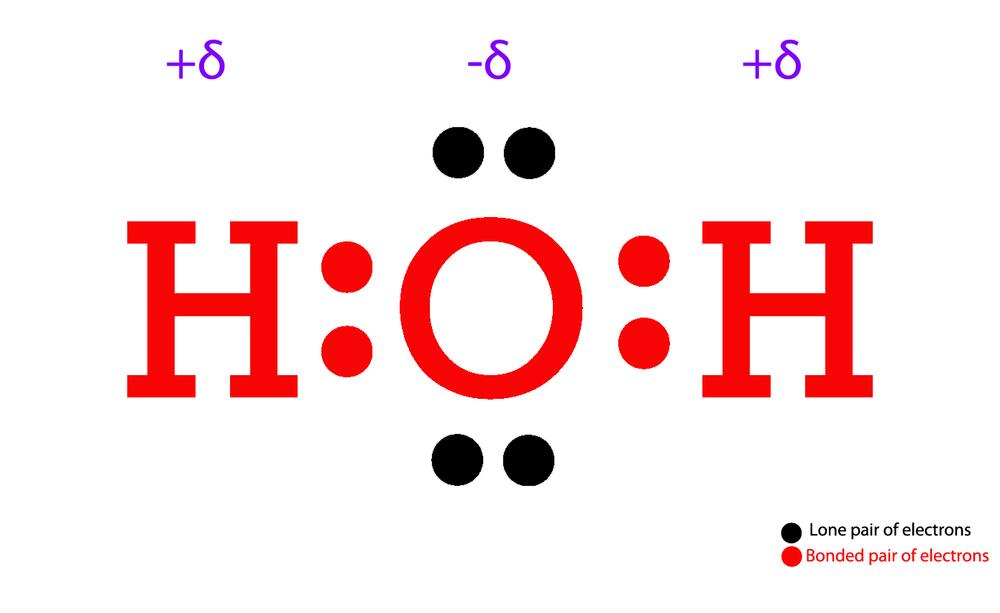
Structure of Water. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). The oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms.
Lewis Structure of H2O, water, dihydrogen monoxide YouTube
The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Table of Contents How to draw Lewis Structure for H 2 O Molecular Geometry of H 2 O Hybridization of H 2 O

Lewis Dot Diagram For H2o Free Diagram For Student
A quick explanation of the molecular geometry of H2O (Water) including a description of the H2O bond angles.Looking at the H2O Lewis structure we can see tha.
Lewis Dot Diagram For H2o Free Wiring Diagram
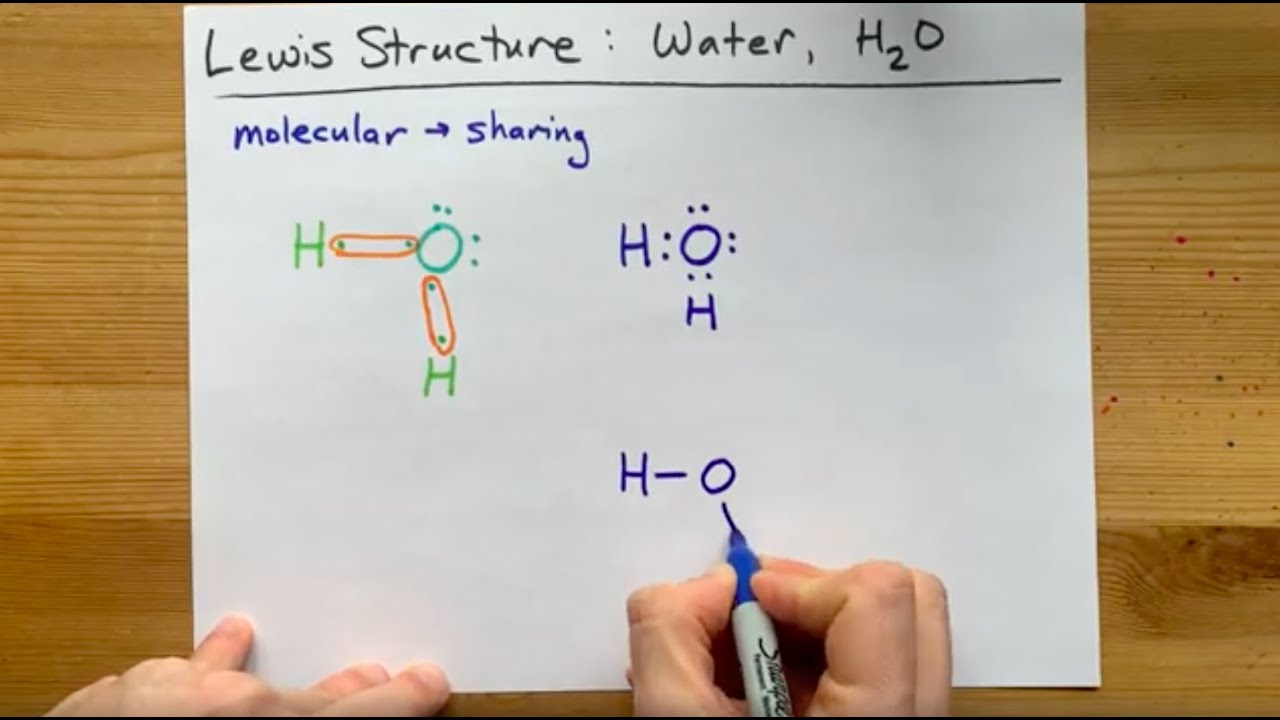
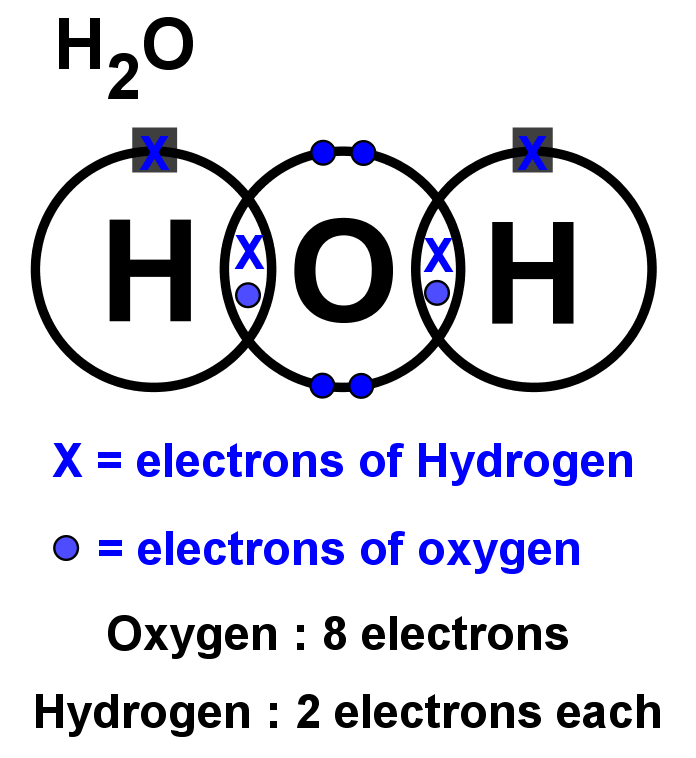
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule.. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. Chemists normally represent a bond using a line instead of two dots. The structures of H 2,.

【4 Steps】H2O Lewis StructureLewis Structure for H2O (Water)Lewis
So starting off by drawing the Lewis structure: H 2 O: Water has four electron groups so it falls under tetrahedral for the electron-group geometry. The four electron groups are the 2 single bonds to Hydrogen and the 2 lone pairs of Oxygen. Since water has two lone pairs it's molecular shape is bent. According to the VSEPR theory, the electrons.

H2O Lewis Structure, Molecular Geometry, and Hybridization
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen.
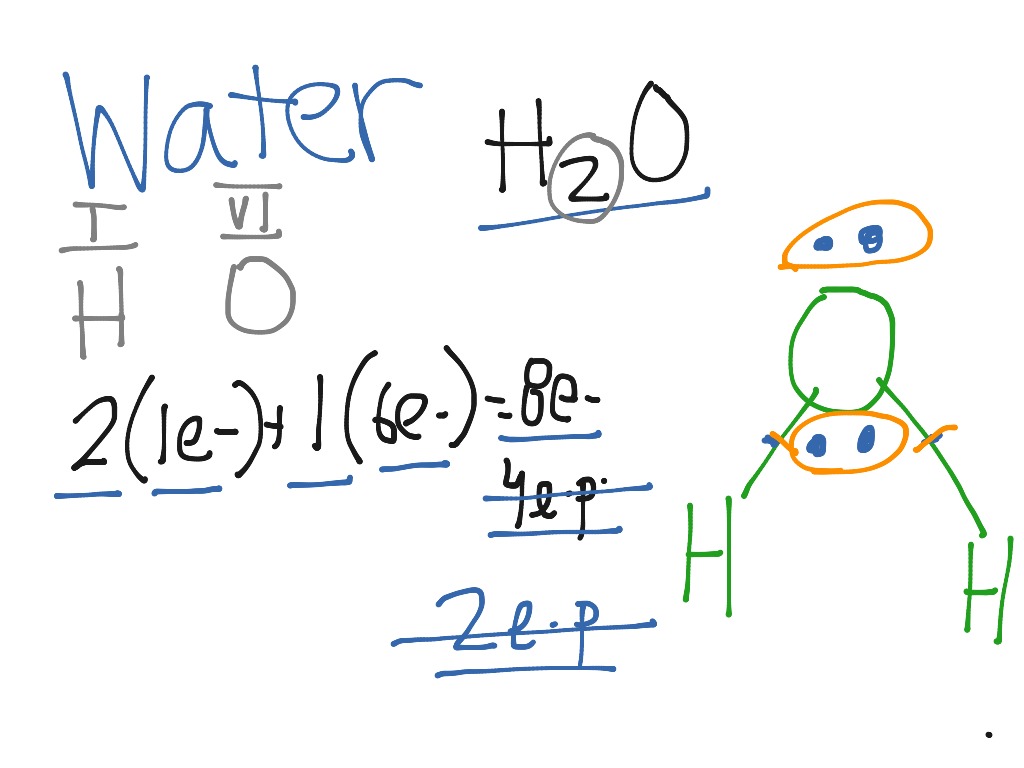
Lewis Dot Diagram For H2o Wiring Diagram
The Lewis Structure for water is useful because it allows to determine the molecular geometry and the polarity of the molecule. Because of the two lone pairs, H 2 O will have a bent molecular geometry and it will be a polar molecule. Remember that Hydrogen only needs two electrons to have a full outer shell.

H2O Lewis structure and Molecular Geometry [No1 Best Explanation
In the Lewis structure of H2O structure there are a total of 8 valence electrons. H2O is also called Water. ---- Steps to Write Lewis Structure for compounds like H2O ---- 1. Find the.

In this video we are going to learn about the Lewis structure of H2O
Lewis Structure of H 2 O indicating bond angle and bond length Water ( H 2O) is a simple triatomic bent molecule with C 2v molecular symmetry and bond angle of 104.5° between the central oxygen atom and the hydrogen atoms.
H2o2 Dot Diagram
1) Elementary models: The Lewis structure predicts that two lone pairs are (a) localized on the oxygen atom of water and that (b) both lone pairs are equivalent. The Lewis structure, combined with Valence Bond Theory, would predict that lone pairs occupy two equivalent hybridized \(sp^3\) atomic orbitals on oxygen.
Lewis structures StudyPug
In the lewis structure of H 2 O, there are two single bonds around oxygen atom. Hydrogen atoms are joint to oxygen atom through single bonds. Also, there are two lone pairs on oxygen atom. Water molecule is a simple molecule. Drawing lewis structure of water molecule is simple than some of other complex molecules or ions.

Lewis Dot Diagram For H2o Wiring Diagram
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.